In this guide, you`ll find out:
Overview
A UK-based clinical genomics lab worked with Automata to introduce flexible, scalable lab automation, enabling them to reduce manual intervention in the preparation of samples for next generation sequencing (NGS). Automata’s solution will result in a significant reduction in manual interactions, and an increase in output per square foot of lab space and per staff member.
The brief
The clinical genomics lab performs an extensive repertoire of genomic tests using NGS, providing accurate profiling of solid tumours and haematological malignancies which can lead to targeted therapy and immunotherapy.
An increasing number of therapies are approved to treat cancers with specific genomic biomarkers, and genomic testing is recommended for all patients with a metastatic or advanced cancer that can be targeted in this way.1 This means that certified laboratories anticipate a rising demand for their services.
In this case, the lab was not able to increase capacity by adding more staff, because of limited availability and space restrictions. The existing workflow involved disparate pieces of equipment, requiring manual transfer of samples and data from one workstation to another, increasing the risk of human error and minimising walkaway time.
The lab approached Automata to understand how they could use automation to increase throughput capacity within their existing lab space, with minimal additional staff. Initially, the lab wanted to be able to process approximately 50,000 samples per year – three times their current capacity of 16,000 samples.
Since future-proofing their set up was a priority for the lab, it was important that the automated system they used would grow and develop as their needs evolved. This required a solution with built-in flexibility, which could scale capacity over time, and which fitted within the existing lab space.
The importance of the samples processed by the lab meant that traceability was critical, with increasingly stringent audit trails required, and new staff would need to be able to use the system with the minimum of training.
The challenge
Finding an automation solution that did not require additional space or staff to increase capacity
Key challenges in this project included:
- Multiple sample types: the lab screens DNA and RNA from both blood, saliva, and tissue samples, and pre-extracted DNA/RNA samples, so the system needed to be able to process multiple sample types and containers
- Contamination risks: Ideally, the process which generated the DNA/RNA libraries should be physically separated, to reduce the risk of amplicon contamination of the samples.2 This meant splitting the tasks performed by the existing liquid handler
- Scalability: the workload of the lab is expected to increase over the next few years, so the automated system needed to exceed the current capacity of the lab, and be flexible enough to scale up in the future
- Space limitations: Increasing lab space and recruiting more staff were not possible, so a solution was needed that could increase throughput without requiring additional laboratory space or staff.
Automata's solution
Working with the lab’s Senior Scientists, Automata optimised the existing workflow for automated processing, reducing manual interactions
Automata is a lab automation platform that combines specialised lab benches with open, integrated orchestration software. This turns labour-intensive workflows into streamlined automated systems, providing true ‘walkaway’ time for staff. Automata’s solutions are achieved through combined robotic and digital integration of multiple devices, many of which a lab may already have.
Automata worked with the lab to review their specific protocols and build their manual procedures into an automated workflow. Collaborating with the lab’s senior scientists, Automata optimised the existing workflow for automated processing, reducing manual interactions.
Since each piece of equipment could operate concurrently in the automated solution, the capacity of the new system was substantially greater than the existing series of processes.
Built on the Automata LINQ robotic lab bench, the automated system had the capacity for expansion over time to further increase throughput as needed.
Automata’s cloud-based laboratory orchestrator was used to provide a software platform that seamlessly connected each activity in the workflow, providing full traceability for each sample. Barcode scanners on each LINQ module and within the instruments themselves provided tracking for each sample, right down to its position within each microplate.
Automata divided the complete process into two parts – Pre-PCR and PCR/Post-PCR, to reduce the risk of contamination.2
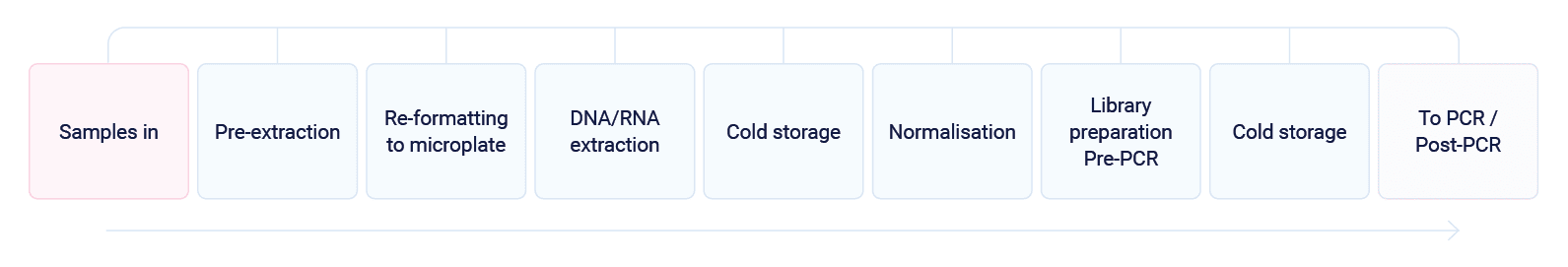
Pre -PCR
Samples received by the lab will be arranged into automation-friendly racks and loaded on to the Automata system transport layer. At this stage, sample tracking is initiated.
PCR / Post-PCR
Staff will take the microplates from cold storage after the Pre-PCR process and load them into the PCR/Post-PCR system.
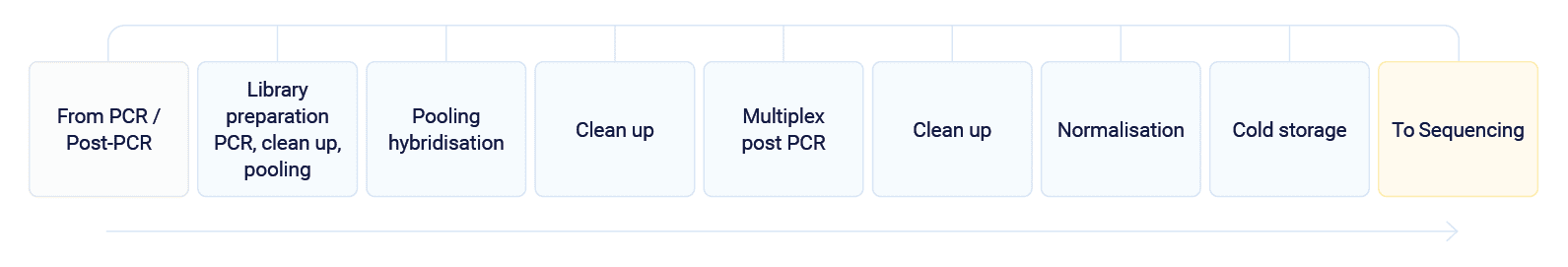
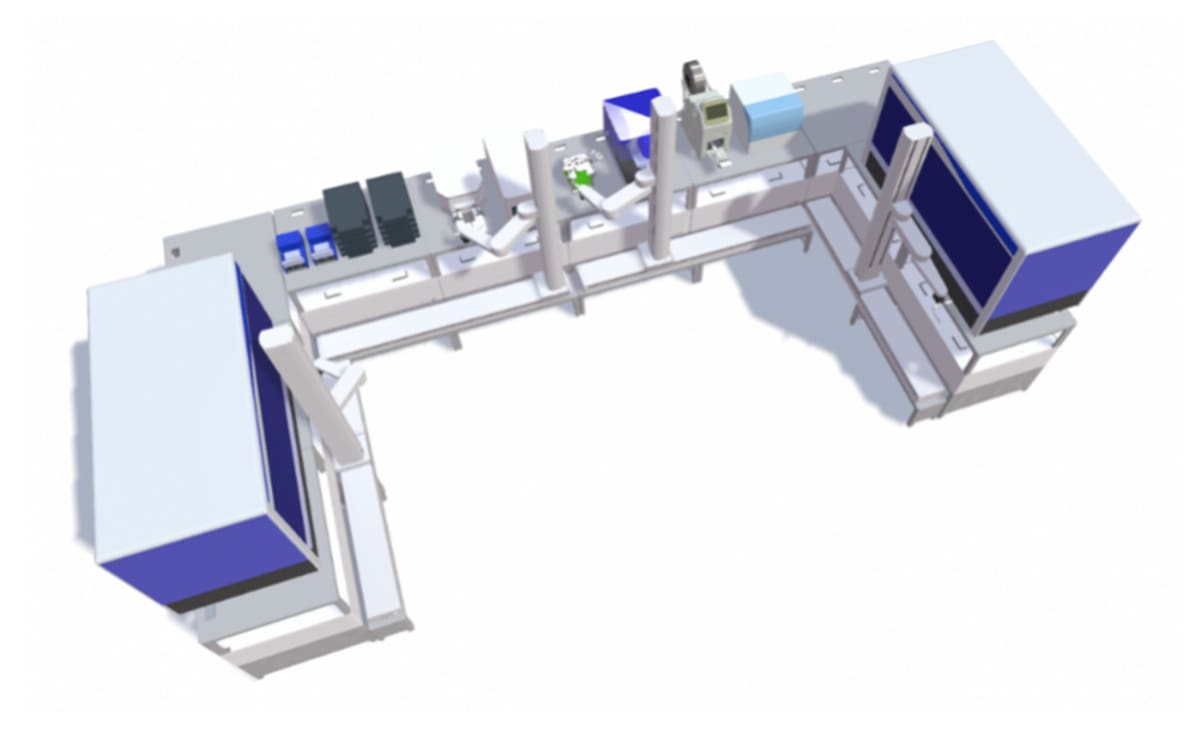
After this point, lab staff will take the microplates from cold storage and transfer them to the sequencing systems.
The following equipment was integrated into the two systems:
| Liquid handlers Plate sealer and peeler Decapper Barcode scanner Microplate incubator Microplate shaker Cold storage unit Microplate vortex | DNA extraction platform Microplate reader NGS system Plate shaker Thermocycler Microplate centrifuge Automata LINQ modules Automata Scara Robots |

Ready to Scale Up Your Lab’s Efficiency?
Unlock seamless, scalable automation with LINQ, connecting instruments and workflows to adapt as your lab grows.
Estimated impact
Based on the final solution that will be installed, the new, fully automated process is expected to achieve:
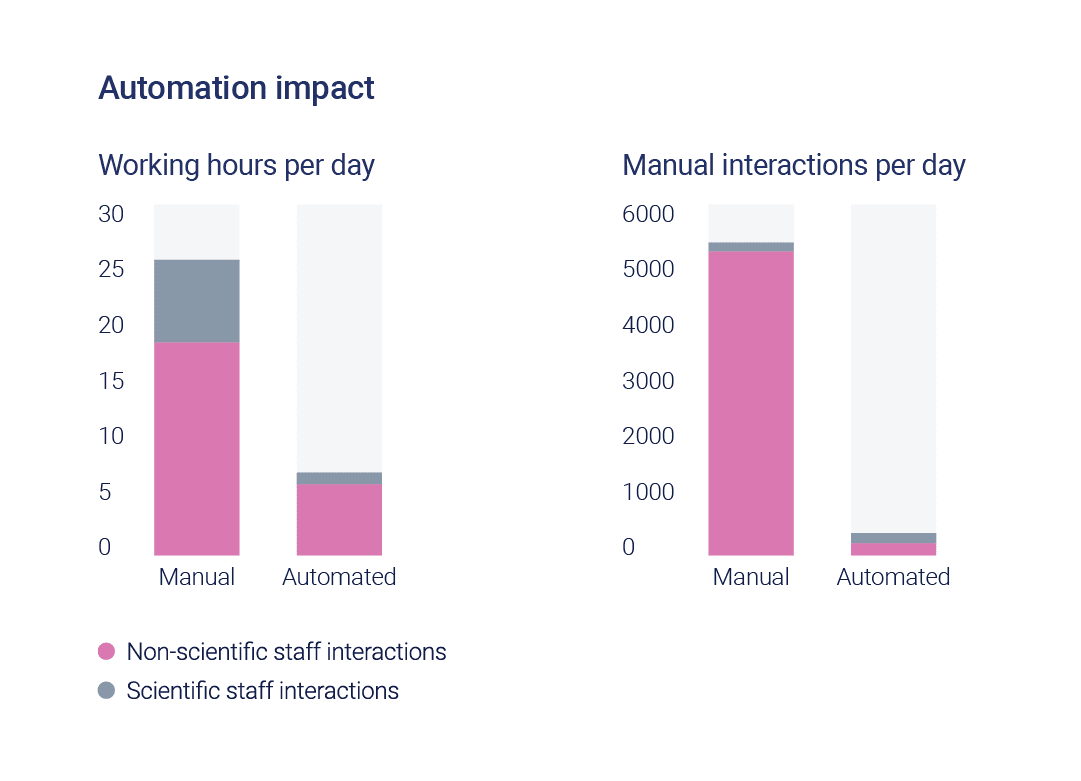
3x increase in output per square foot
3x reduction in staffing hours
Substantial reduction in manual interactions
Reduced risk of manual errors
Increased data quality as all actions will be fully traceable