Find out how automation can accelerate the ELISA development process and help your lab deliver results at scale.
What is ELISA development?
The enzyme-linked immunosorbent assay (ELISA) is a quantitative assay commonly used in biological research, drug discovery, and clinical diagnostics to measure the levels of a target antigen in a biological sample 1.
The need to identify a specific target requires a new assay to be developed each time a novel target needs to be quantified.
What are the steps in ELISA development?
ELISA development consists of three main steps 2:
- Assay design: The first step of ELISA development is to design the assay. Since there are several types of ELISA (direct, indirect, sandwich, competitive, and ELISpot assays), each format should be considered, and the best assay format determined, based on experimental requirements. Then, the required components can be gathered, and a working protocol can be developed
- Assay optimisation: The next step, assay optimisation, involves systematically adjusting each variable and testing it until each variable and component of the assay has reached optimal conditions. For example, antibody, sample, and buffer concentrations should be tested to ensure a good signal-to-noise ratio
- Assay validation: Finally, the newly designed and optimised assay must be validated to ensure results are robust and accurate, and to produce documentation as evidence that the method fulfils the requirements for its intended use 3
How long does it take to develop an ELISA?
- Existing resources: The timeframe of ELISA development will be primarily determined by the purpose of the assay and the availability of existing resources. For example, if the ELISA is aimed at measuring an entirely new target, validation will take far longer than if an existing assay can be adapted for a slightly different purpose e.g. a different sample type. Similarly, the availability of high-quality, fully validated antibodies against the target of interest is a determining factor in the time taken to optimise and validate a novel assay.
- Optimisation factors: The optimisation stage of ELISA development can be very time-consuming and labour-intensive, particularly if the assay design is complex and involves many variables. The number and complexity of the components involved in the assay will impact development time, as will the capacity to test several components at the same time. The simpler the assay design, the quicker and easier the optimisation process.
- Validation procedure: Similarly, validating a newly developed assay can be laborious and time-intensive. Validation can be particularly taxing if an assay will later be used in clinical, diagnostics, or drug development settings, due to the requirement to be comprehensively validated and meticulously documented.
- Resources and expertise: The resources and expertise available contribute to the development timeframe. ELISA development is a labour-intensive process requiring considerable money, resources, and staff effort, which can represent a significant hurdle in smaller laboratories.
In summary, the time taken to develop an ELISA can vary significantly depending on several factors. However, it is a labour-intensive, time-consuming, and expensive process regardless of the specifics, and it is crucial to find ways to optimise and improve the efficiency of ELISA development.
How can automation help with ELISA development?
Laboratory automation represents a promising solution to the inefficiencies of the ELISA development process, with several benefits over manual development processes 4.
Speed, efficiency and scalability
Automating ELISA assay development accelerates the process, reducing the time to achieve a fully validated assay. This is particularly beneficial when the assay is required urgently, as it accelerates the time to insight 5,6. Moreover, automation improves process efficiency and relieves the burden of laborious, repetitive tasks such as pipetting serial dilutions from highly skilled scientists, freeing them up for other tasks and improving productivity and output. Furthermore, automating ELISA workflows increases their scalability, facilitating simultaneous variable testing during assay optimisation and further accelerating the workflow.
Enhanced accuracy and precision
During assay optimisation, scientists systematically change each component and test the impact. This involves tasks like serial dilutions, which are notoriously prone to errors. ELISA automation reduces human interaction, reducing the risk of error and improving consistency for enhanced assay precision and accuracy.
Standardisation
Automated systems, such as Automata’s LINQ, perform tasks in a highly standardised, consistent manner, which eliminates the inconsistency issues that often arise when experiments are performed by several different users. This is crucial for validation, ensuring the assay yields robust, reproducible results.
Documentation
Integrating automated hardware and software systems allows data to be automatically collected, stored, and used to generate reports. This is particularly important if the lab is required to comply with stringent regulatory requirements. Moreover, automated software systems such as LINQ Cloud prevent data loss and transcription errors, providing further data quality assurance.
Cost-benefit
Despite the relatively high purchase and installation costs for automated systems, they generally offer significant cost benefits in the long term, owing to their impact on the efficiency and scalability of processes.
LINQ ELISA
Automata’s fully automated systems offer a comprehensive solution for ELISA assay development from design to validation.
Check out our lab automation platform to learn more about how your lab can fully automate your ELISA workflows to accelerate development processes.
Hands-Off ELISA Automation
See how end-to-end automation can reduce manual steps, scale effortlessly, and enhance data integrity in your ELISA workflows
References
1. Engvall E. The ELISA, Enzyme-Linked Immunosorbent Assay. Clin Chem. 2010;56(2):319-320. doi:10.1373/clinchem.2009.127803
2. Minic R, Zivkovic I. Optimization, Validation and Standardization of ELISA. In: Mózsik G, ed. Norovirus. IntechOpen; 2021. doi:10.5772/intechopen.94338
3. Andreasson U, Perret-Liaudet A, Van Waalwijk Van Doorn LJC, et al. A Practical Guide to Immunoassay Method Validation. Front Neurol. 2015;6. doi:10.3389/fneur.2015.00179
4. Bock JL. The New Era of Automated Immunoassay. Am J Clin Pathol. 2000;113(5):628-646. doi:10.1309/DUDM-3Y6L-3R1L-QP15
5. Lagousi T, Routsias J, Spoulou V. Development of an Enzyme-Linked Immunosorbent Assay (ELISA) for Accurate and Prompt Coronavirus Disease 2019 (COVID-19) Diagnosis Using the Rational Selection of Serological Biomarkers. Diagnostics. 2021;11(11):1970. doi:10.3390/diagnostics11111970
6. Gao Z, Shao JJ, Zhang GL, et al. Development of an indirect ELISA to specifically detect antibodies against African swine fever virus: bioinformatics approaches. Virol J. 2021;18(1):97. doi:10.1186/s12985-021-01568-2

Find out how open, integrated automation can accelerate the ELISA validation process…
Read more ELISA assay validation: how automation can help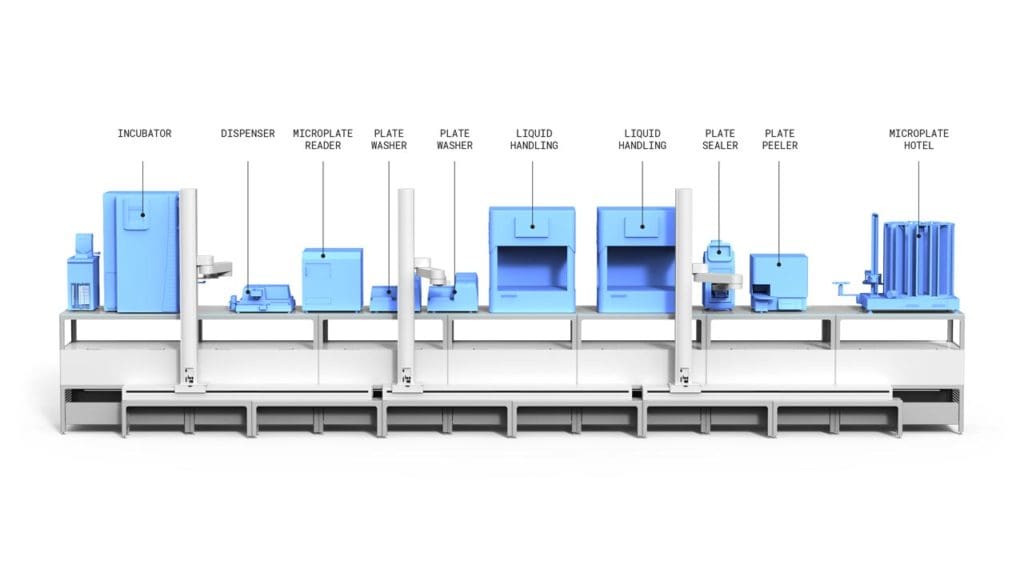
Overview A virology contract research organisation (CRO) worked with Automata to take…
Read more Advancing with end-to-end automation
How Automata worked with a UK-based CRO to reduce human steps and…
Read more Reducing human interaction in ELISA