Jump to:
– Preparation errors
– Timing challenges
– Throughput blockers
– Data inaccuracy issues
– Impact of automating ELISA
– Explore ELISA automation with our team
Enzyme-Linked Immunosorbent Assay (ELISA) is a cornerstone technique in immunology for detecting and quantifying various substances such as peptides, proteins, antibodies, and hormones.
While manual execution of ELISA assays is still common in many research and diagnostic labs, it is prone to human error, leading to inconsistent results.
This blog post discusses frequent mistakes made during manual ELISA assays and highlights how automation can mitigate these issues.
Preparation errors
Inaccurate mixing of reagents and pipetting and cross-contamination
Manually executing critical tasks like reagent mixing and pipetting leaves assays open to mistakes, inaccuracies and inconsistencies from the beginning.
For example, improper mixing of reagents can lead to uneven distribution, while manual pipetting often results in inconsistent volumes. Cross-contamination between wells can also occur due to poor pipetting practices or splashing.
These kinds of pipetting errors affect assay sensitivity and specificity, increase waste and associated costs, and compromise repeatability, while executing manual tasks like this is the leading cause of repetitive strain injuries in the lab
Pipetting is one of the easiest, most cost-effective processes to automate, and as such, is one of the first to be switched from manual to automated.
Read our complete guide to automated pipetting for more information about choosing an automated system and going beyond pipetting to automating full liquid handling processes or even entire workflows end-to-end.
Automated full liquid handling systems speed up the movement of small and precise volumes of liquids and can be programmed to set protocols for aliquoting, mixing, and serial dilution of liquid samples. Compared to their traditional manual counterparts, they increase efficiency, productivity, and cost savings while supporting the delivery of highly accurate and repeatable assay results without researcher-to-researcher variability.
Timing challenges
Inconsistent incubation and reagent addition times
Timing is crucial in ELISA assays because each step — coating, blocking, washing, incubation with samples and detection antibodies, and substrate reaction — requires precise durations to ensure optimal binding and reaction conditions.
Small, often undetectable inconsistencies in incubation start and stop times, reagent addition and even plate-washing processes can result in variations in antigen-antibody binding and enzymatic reactions.
Automating ELISA allows every process step to be controlled, with systems adhering to strictly prescribed incubation parameters and actions for uniform timing across all samples.
And while automating these processes removes manual errors and strain injuries, it also frees scientists from the frustration of closely monitoring assays.
Throughput and productivity restrictions
Too much scientist dead time
ELISA systems can be restricted by incubation time requirements, washing and immunostaining requirements, and shift availability.
Automated ELISA systems don’t necessarily work faster than a person, but they allow machines to be tended to concurrently and enable complete hands-off time as the system can run independently of human interactions.
Because of this, automation is an excellent way for labs to increase productivity and reliably and flexibly scale throughput.
Data inaccuracy problems
Reproducibility, misinterpretation and validation consequences
Manual data recording within any setting is prone to transcription errors, and given their sensitivity and specificity, maintaining the integrity of the data throughout the ELISA process is paramount.
Consequences of inaccurate data recording in ELISA include:
- Misinterpretation of results
Incorrectly recorded data can lead to the wrong conclusions about the presence or concentration of a target molecule, affecting downstream applications, such as diagnosing diseases, evaluating vaccine efficacy, or conducting basic research.
- Reduced reproducibility
If data is inaccurately recorded, other researchers or even the original team may struggle to replicate the findings, undermining the study’s credibility.
- Compromised quality control
ELISA assays often include controls to ensure they are working correctly. Inaccurate recording of control data can obscure issues with the assay, such as reagent problems or procedural errors, leading to erroneous results being accepted as valid.
- Erroneous statistical analysis
Statistical analysis relies on accurate data input. Incorrect data can skew statistical results, leading to false positives or negatives and ultimately flawed scientific conclusions.
- Wasted resources
Inaccurate data recording can necessitate repeating experiments, leading to unnecessary consumption of resources and time.
- Regulatory and compliance issues
Accurate data recording is often a requirement for regulatory compliance, especially in clinical settings. Inaccurate records can result in regulatory non-compliance, potentially leading to legal consequences and loss of certification or accreditation.
Automated LIMS
Companies are already implementing Lab Information Management Systems (LIMS) alongside automated laboratory machinery to revolutionise workflows and reduce the potential for data errors.
The LINQ Cloud software bridges the physical and digital aspects of automated ELISA on our lab automation platform, LINQ. It integrates with all the instruments in use, transferring data to any LIMS in real-time without manual transcription. This enables labs to produce high-quality, reproducible data sets for contextualisation, standardisation, collaboration, and easier validation.
How can automation help with ELISA validation?
Validation is a crucial part of the ELISA process and is required to ensure the workflow’s precision, accuracy, and reproducibility. It assures users and regulators that assay results are consistent and reliable.
Automated ELISA platforms can help validation with precision, standardisation, speed and efficiency, throughput, data collection and analysis, and compliance with regulatory requirements. Read more about ELISA validation and automation and discover the best practices in error handling.
Conclusion
While manual execution of ELISA assays is common, it is fraught with potential errors that can compromise results. Automation offers a robust solution to these issues, enhancing accuracy, consistency, and efficiency in ELISA assays. By integrating automation into your laboratory workflow, you can significantly improve the reliability of your results, ultimately advancing the quality of your research and diagnostic capabilities.
How we helped one CRO automate ELISA to achieve amazing results
We worked with a virology contract research organisation (CRO) to design a fully automated ELISA system to maximise throughput, provide robust data management and significantly reduce manual interactions.
80 – 95.25%
reduction in manual interactions
Up to 530
microplates per day throughput
Reduced risk
of manual errors
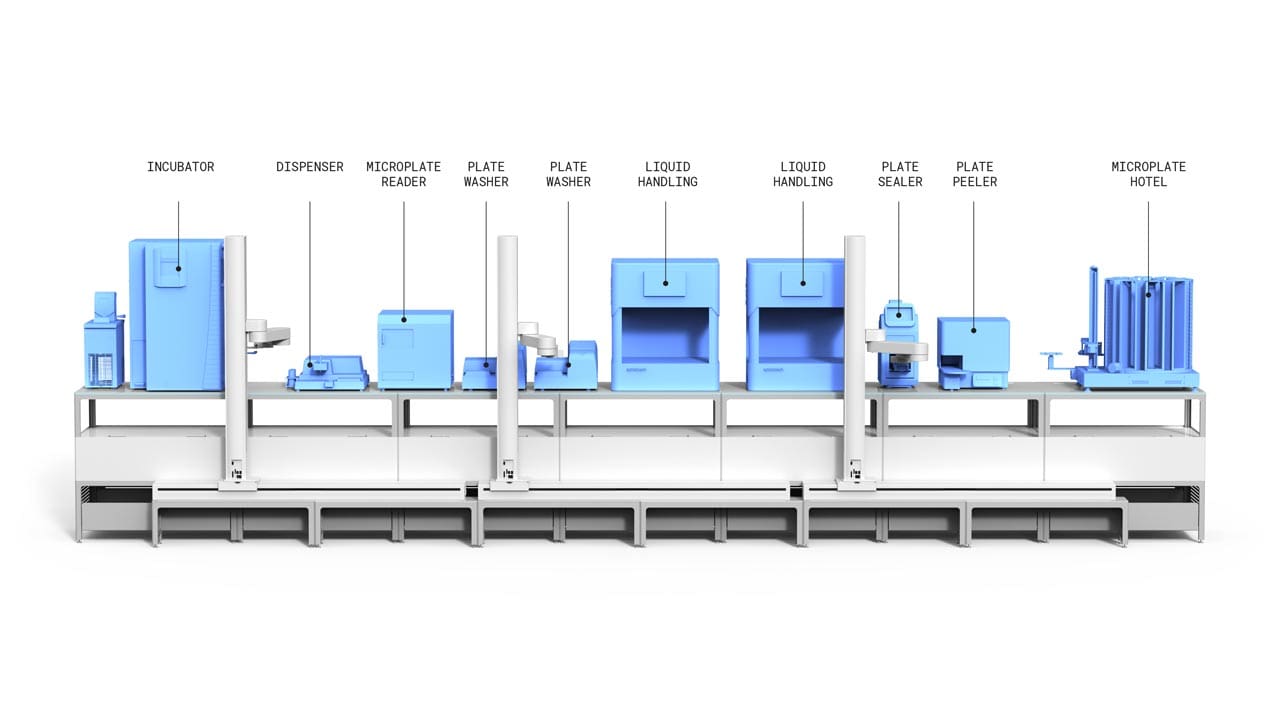
Built on the Automata LINQ robotic lab bench, this ELISA system has equipment operating concurrently, unlocking a lab’s maximum capacity. This can be expanded or adapted over time, with additional lab equipment or adding further LINQ modules.
The cloud-based Automata LINQ software seamlessly connects each activity in the ELISA workflow, providing full barcode scanning traceability for each sample, and adding test data to the lab’s LIMS system.

Find out how open, integrated automation can accelerate the ELISA development process…
Read more Scaling up ELISA development with automation
Find out how open, integrated automation can accelerate the ELISA validation process…
Read more ELISA assay validation: how automation can help
We look at what causes errors in biology lab experiments and how…
Read more Common sources of error in biology lab experiments