

How we made a 6-hour cell culture assay into a 70-minute process
Why taking learnings from manufacturing – and taking automation ‘off deck’ – resulted in a 5x increase in one biotech lab’s throughput
It’s clear that the impact automation has made to the manufacturing industry has yet to be realised in the lab space.
The problem is, many of the labs who have adopted automation are not making the most of it. They are still working in batch processes with a method built from the bench and scaled up, rather than using workflows that are properly optimised for their bottlenecks or for the flow of materials through their system.
As a result, even with automated lab equipment in place, people with PhDs, at the forefront of their respective fields, are spending their days moving microplates and struggling to scale their experiments to achieve the world-changing results they should be getting from automation.
The manufacturing industry has long used automation to parallelise their workflows, allowing processes such as formulation, blending, packing and cleaning take place simultaneously, increasing efficiency.
If labs are able to take these lessons from manufacturing, they can utilise automation not only to improve the quality of results, but also to improve efficiency and better utilise staff by removing repetitive processes.
This was the approach we took with a recent client, who, for legal reasons, we’ll refer to as ‘Super Cool Biotech’ (SCB for short). SCB was able to turn an almost 6-hour cell culture process into a 70-minute one by parallelising their processes, taking processes off deck and better connecting their equipment.
Let’s take a look at how SCB achieved this incredible result.
How and why SCB failed to reap the benefits of automation
Now, SCB are doing some exceptionally cool things, but since we need to keep those private (for now) we’ll talk in broad strokes.
When we first met with them they were using a Big Box Liquid Handler (BBLH)™ (vendor anonymised to keep lawyers on side). And that BBLH™ worked well – for what it was designed for. It took their liquid handling process, and made it accurate and repeatable in a way that you never achieve on bench-top with handheld pipetting.
However, they needed to scale their throughput. The trouble was that, despite the fact SCB had integrated automation, the equipment was unable to meaningfully scale their operations.
This inability to scale was the result of a few fundamental limitations to these systems:
1. The amount of processes they can do are limited by deck space. When they reach the limit of that deck space, the only answer is to add more BBLH™’s – which in a lab with limited space was simply not possible
2. They view workflows in a very linear manner, meaning that processes have to happen sequentially, rather than in parallel
3. Their capabilities are dictated by their catalogue of on-deck products. This means they had to use equipment that was compatible with BBLH™, rather than being free to use the equipment that worked best for them.

Automated liquid handling was not enough
SCB had a problem. While their quality had vastly improved by integrating the BBLH™, given the limitations of the product ecosystem, they could only run 60% of their workflow on the system. This meant they were unable to integrate freezing and centrifugation steps onto the deck of the BBLH™. And because of that limited deck space, they could only fit minimal incubators on deck. Since this was a critical stage in their process, this meant the BBLH™ (a very expensive and highly capable piece of kit) sat unused for hours as lab workers waited on incubations to finish.
As a result of the time it took to wait for other processes to complete, it took 5 hours and 55 minutes to automate a process that ran for only 3 hours on the bench top.
They needed a better way.
They needed their automation equipment to work smarter.
Making SCB’s automation work ‘smarter’
At Automata, we approach lab automation in three stages. This was how we worked with SCB:

Stage one: We took processes off-deck – and optimised SCB’s bottlenecks
An incredible, expensive liquid handling system should never be waiting on other, cheaper pieces of equipment to finish before running. Spending hundreds and thousands of dollars on a-state-of-the-art liquid handler and then letting it wait while plates run through thermocycling or incubation stages is like buying a Formula 1 car for your weekly grocery shop.
In the case of SCB, we took the incubation off deck and immediately removed the deck of the liquid handler as our limiting factor. By taking dispensing tasks away from the BBLH™, these processes became lightning quick. A Certus Flex or a Multidrop can bulk dispense reagents much faster than an eight channel pipette and a reservoir.
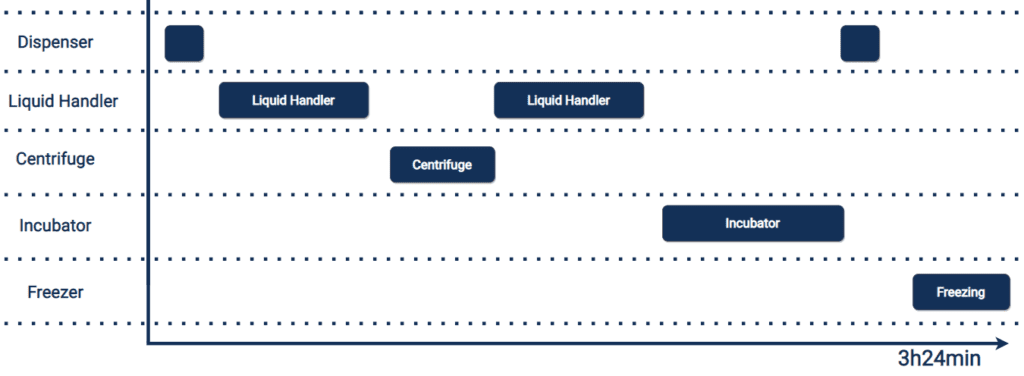
Stage two: We parallelised the workflows
By moving processes such as incubation off deck, we could move away from an inflexible linear assay which involved many hours of waiting time, to a multi-tasking asynchronous workflow.
In the new, parallel workflow the BBLH™ handled liquid, while bulk dispensers dispensed reagents and specialist high capacity incubators held a large capacity of plates to incubate concurrently.
Rather than each process being done, one after the other, in an inefficient manner, all the processes instead happened in harmony. It was the equivalent of moving from one person trying to play every instrument in the orchestra, to all the instruments being played at the same time – harmony.
This harmonious parallelisation of the workflows allowed the lab to do more at a given time. And opportunities to scale up throughput are opened up.

Stage three: We connected the equipment
Finally, connecting SCB’s equipment so it could work together was critical in order for the parallelisation process to work effectively.
The problem with much of the automated lab equipment on the market today is that it cannot work in tandem with equipment from other providers. This can be one of the biggest challenges for labs wanting to automate entire workflows.
At Automata, we are device agnostic and work with a range of lab equipment, connecting it together using robots to transfer labware between equipment. Our software connects all of the equipment together, allowing scheduling and data management to all happen on one screen.
Connecting the equipment in this way took SCB from a partially automated process, into a fully automated one through the power of connected equipment.
How to bring the principles of manufacturing automation into your lab
It’s this approach that we bring to every lab visit we go on, every SOP we review, every time we sit in the corner of a facility with a stopwatch and a clipboard.
It’s important to say that these stages don’t always offer this rate of change – for some processes there’s a perfect piece of equipment already on the market. But by using these stages we’ve laid out, you can look at your workflow differently and start to see if you can reduce manual interactions and increase throughput, while using less space.
Here’s how you can bring these stages to your lab:
1. Optimising bottlenecks – Your most expensive piece of equipment should (almost) always be your bottleneck. For example, if your liquid handler is waiting on a plate reader, a thermocycler or an incubator, you should look into how you can optimise its usage
2. Parallelisation – Processes run in tandem are always going to be more efficient. Consider taking long processes off deck in order to optimise the full workflow.
3. Connection – To truly realise the benefits of parallelisation, you need your lab equipment to be connected. Fortunately, there’s an exciting new wave of software products that you can use to build a more connected lab. Hardware offerings are beginning to catch up, too.
The impact of taking on learnings from manufacturing
By taking on automation learnings from the manufacturing industry, SCB was able to revolutionise the way their lab operated.
For a start, that long workflow got a lot quicker, going from 5 hours and 55 minutes down to 1 hour and 12 minutes.
SCB’s ability to scale was also vastly improved, and they were able to achieve 5x the output they had been able to when we first began working with them.
Investing in another BBLH™ would not have been possible, as they did not have the space in the lab. Yet this level of automation density allowed them to do more in their existing space.
Finally, moving to a fully connected system that can do 100% of the workflow as opposed to 60%, the SCB achieved full walkaway time, allowing their team to do more really cool science and less microplate management.
Learn more about how we can work with you to optimise your lab’s workflow.
