The challenges
Cell Line Development (CLD) is one of the most utilised processes in modern cell biology.
Whether for biotherapeutics, new technology research, or new screening platforms for cell-based assays, developing high-producing, stable cell lines has become a fundamental part of scientific discovery. But there remain challenges in CLD.
Automata’s open integrated automation (OIA) solution tackles scale, reproducibility and traceability issues in CLD, allowing end-to-end automation that doesn’t compromise flexibility.
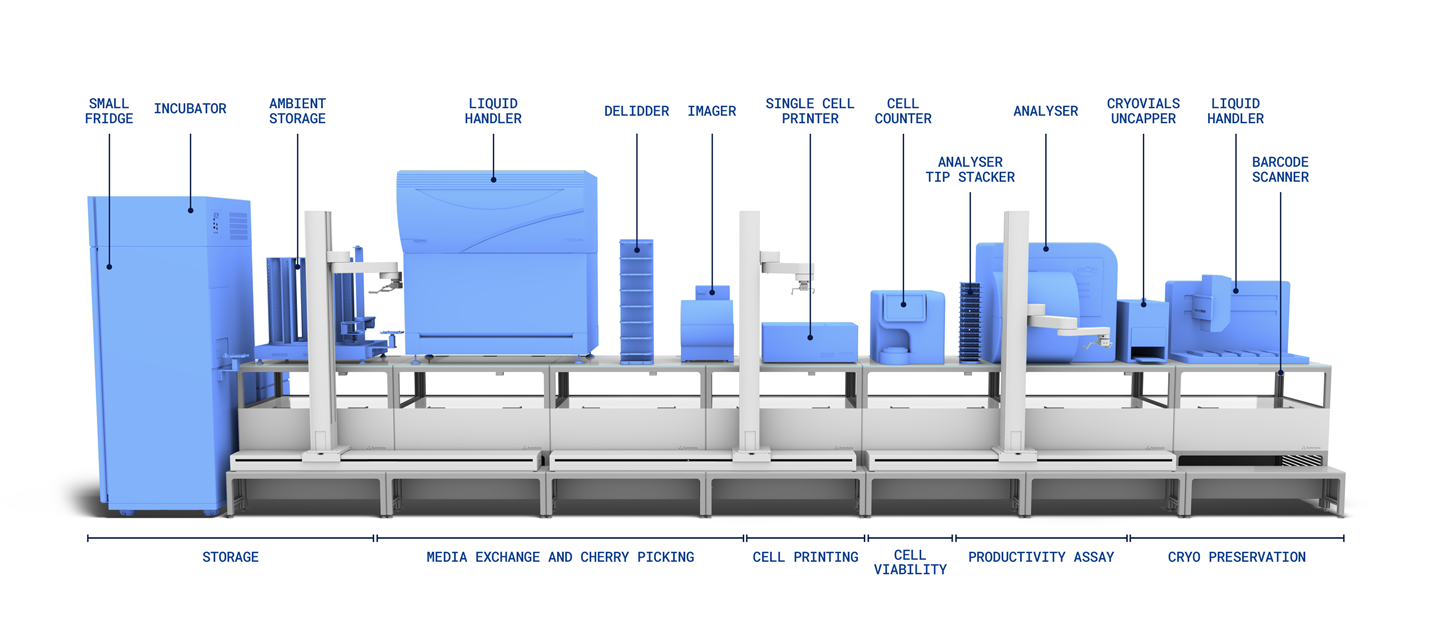
Our open integrated automation platform, LINQ, combines three components to create a lab automation solution that works for you: the Automata LINQ bench, the Automata LINQ software, and a world-class service.
Integrating with most instruments and featuring a modular design, the Automata LINQ bench is easily adaptable for any workflow or any existing lab space.
Unlocking scale
CLD is essentially a numbers game; the more clones you’re able to analyse, the more chance you have of isolating high-performing cell lines with the characteristics you desire. But many conventional approaches to CLD make decisions based on a relatively small dataset.
For example, when the selected clone is passed to process development in biotherapeutics, the starting data points are typically very minimal and the number of clones is small. With automation, and higher-density labware, you are able to seed into many 96 or 384 well plates.
High throughput whole well imaging and characterisation enables the generation and screening of large numbers of clonal cell lines simply not possible without automation.
Enhancing data and traceability
With end-to-end automation, it also becomes possible to automatically unify data from multiple sources and instruments with full traceability. Complete datasets tied to clone identifiers can be built and reported back to recording and analytical tools within the digital ecosystem.
For biologics producing cell lines, this level of data integrity and traceability forms a key part of Clonality Reporting for Food and Drug Administration Investigational New Drug proposals, for example.
Automation also opens the possibility of layering a multitude of analytical steps that would be unachievable manually, allowing decision-making based on data sets with greater dimensionality.
It becomes possible to include clonality, flow cytometry, product quantitation, and characterisation, along with all the methodology metadata, so a complete dataset can be built around your developed cell line early in the process.
Complete workflows
From cell printing and imaging, through productivity screening and expansion to harvesting and master cell bank creation, it is possible to build a complete end-to-end cell line development platform as one holistic workflow.
Is Automata right for my cell line development lab?
Here are a few answers to some frequently asked questions
Yes, in most cases. If the analytical device is automation friendly, it can likely be integrated. We have worked with different imaging systems, plate readers and other analytical tools used in cellular biology.
The LINQ platform itself is compatible with a wide range of labware, including 384 well plates through to 1 well plate, and other types of labware too. As part of any system design, we’ll also ensure that the devices integrated are suitable for the required labware.
With LINQ it is expected that some labs will start with processes that are in greatest need of automation, and then expand.
Expansion is typically through a combination of adding benches, and/or devices.
If your system is enclosed we also plan out what that expansion might look like so that we can consider what the growth of the containment will look like in parallel.
There are several options for keeping a sterile work environment, dependent on your needs. We can utilise the containment offered by the liquid handler manufacturer, create a larger high-efficiency particulate air (HEPA) filtered laminar flow environment, or a full biosafety level (BSL2) enclosure.
The LINQ bench allows for workflows including the reformatting of plates and clones, as well as media exchange and the passaging of cells for extended cell growth protocols.