Enhance precision, increase throughput and free up valuable time
Automating NGS library preparation
Library preparation is involved in any number of next generation sequencing (NGS) processes, from RNA sequencing to cancer panels. It often takes highly trained scientists a considerable amount of time, creating bottlenecks and a frustrated, underutilised workforce.
Automating library preparation – either as part of an end-to-end NGS workflow or as an independent workcell – is easy with the right solution.
For labs with limited space, or resources, or those looking to increase throughput without adding additional staff or instrumentation, open, integrated automation (OIA) solutions can unlock untapped potential.
LINQ is our first OIA platform and with it, you can create fully automated library preparation workflows with amazing results.
The benefits of automating library preparation

More walkaway time
Fewer deck setups, 99% reduction in manual touchpoints and end-to-end connectivity means LINQ does the work while your lab team innovate.

Simplified workflows
End-to-end automation with LINQ can include extraction, library preparation and quality control with or without separation – LINQ is designed for you.
Optimised resources
LINQ can integrate existing equipment and optimise its use, helping you get the most from your current investments and any you make in the future.
Benchtop automation vs LINQ solution
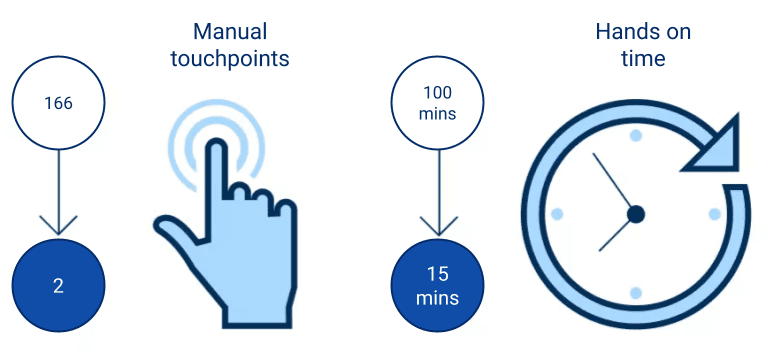
A lab running a daily RNA sequencing protocol using two large liquid handlers, requiring manual attention for plate handling and deck set ups would see manual touchpoints almost eliminated with automated NGS. Deck setups would be reduced significantly and throughput doubled – with no additional liquid handlers needed.
Optimising pre and post-PCR in RNA sequencing
For those running an RNA sequencing protocol daily, within normal working hours, across two pre and post-PCR laboratory spaces with manual plate handling and deck setups, a lab could expect to save time and scale up with no additional instrumentation or staff needed with a LINQ set up like this:
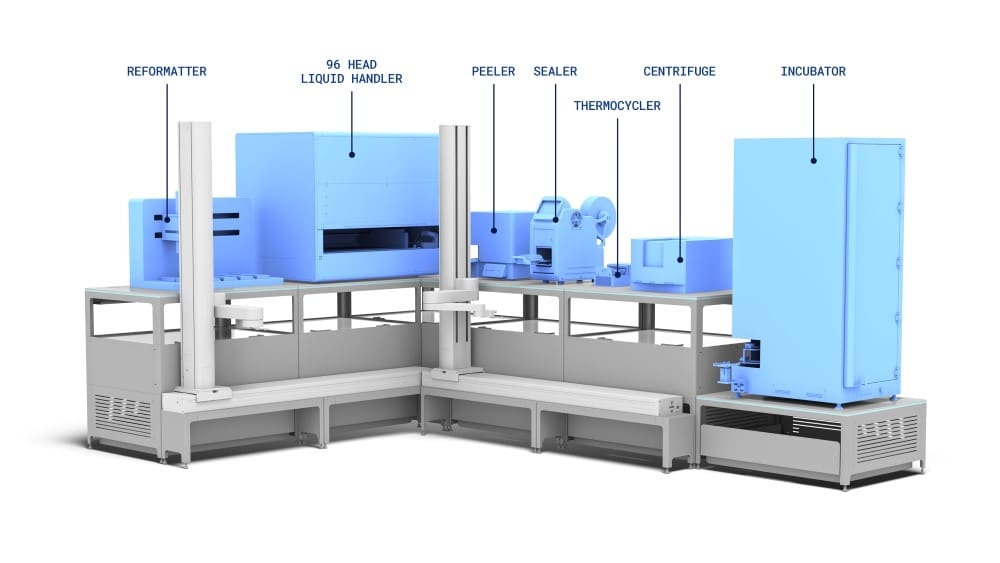
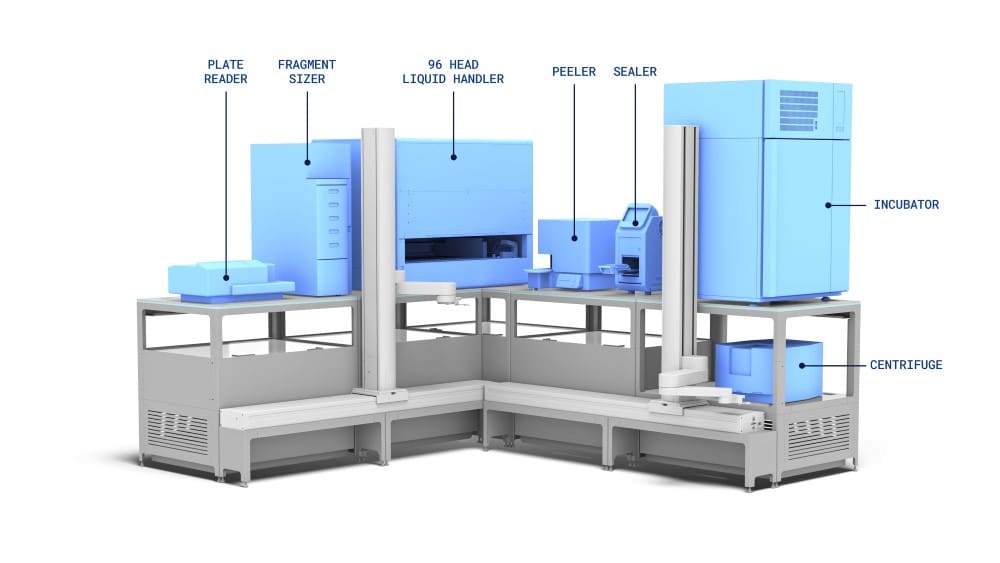
Pre and post-PCR library preparation workcells integrated and automated on the LINQ Bench
The impact of automated library preparation for next generation sequencing
By automating NGS library prep you could save valuable time, eliminate human errors and reduce the number of instruments you require.
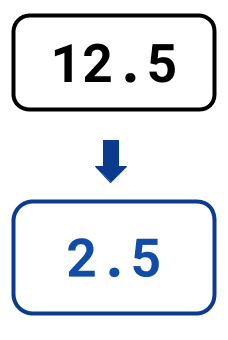
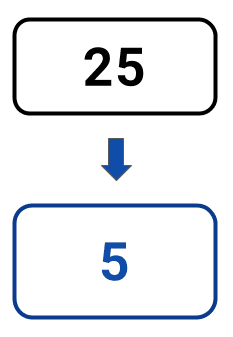
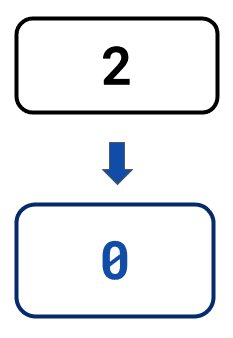
The statistics below illustrate the savings you could make by automating NGS library preparation with LINQ vs. with benchtop-only solutions when seeking to double throughput:
Manual hours
per week
Desk set-ups
per week
Additional liquid
handlers required
Some of our clients
Further reading
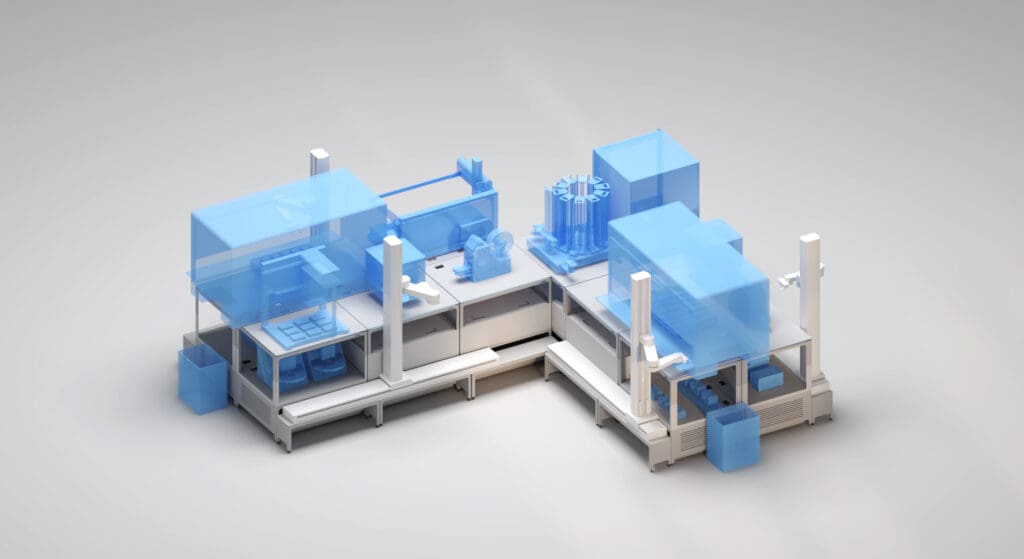
An increased throughput library preparation system with high functionality and adaptability
Read more Sequencing preparation for drug discovery
Automata Senior Application Scientist Alice Tome-Fernandez discusses how to achieve more throughput…
Read more Scaling nucleic acid extraction with adaptable automation
Discover how you can reduce time-intensive processes, improve data quality and optimise…
Read more Improving NGS library prep with automationAutomated NGS library preparation FAQ’s
Here are the answers to some frequently asked questions about library preparation automation
The workcell shown is an example of a workcell capable of running a specific RNA-library preparation workflow. The exact configuration can be customised for optimal running of your workflow.
After initial reagent loading, the workcell will use the transport layer and robot arms to set up the decks as required for each of the library preparation step.
As long as the devices required are on the system then several different workflows can be deployed to each workcell.
By moving highly occupied devices such as thermal cyclers off deck it allows better utilisation of the liquid handlers to do complex liquid handling task, which in turn will increase throughput capacity for complex workflows such as library prep.
Chemistry is dependent on the instruments selected for the system so as long as your chemistry is not tied to instrumentation that is not automation friendly it can be used on this or a similar solution.
The separation of a pre-PCR and post-PCR workcell is completely dependent on your workflow. If you are using a PCR-free workflow or the physical separation isn’t needed a single system can be designed to give a similar throughput.
Yes, the liquid handlers shown in this example can be swapped out for any that are capable of performing the liquid handlers required for your chemistry.
- Walk-away. When benchtop automation is used for library preparation there are often still many manual touch points needed, sometimes across a multiple-day process. An operator may be required to seal and move a plate to another device in order for the process to proceed. There will also be multiple deck setups for each liquid handler. These steps are often an inconsistent time apart which leads to unnecessary downtime for scientists waiting for plate movements and manipulations.
- Scalability. When done manually the only way to scale library preparation methods is to get more skilled scientists to perform the process, while benchtop automation requires getting multiples of large and expensive equipment. These options aren’t efficient or viable for most laboratories and can result in more errors or suboptimal use of people and space.
- Utilising Current Automation. With library preparation being a workflow with high levels of automation adoption already, the next step is to integrate numerous complicated liquid handlers which LINQ can do.
Library preparation describes the process of making nucleic acid samples sequenceable. It’s the method used to prepare RNA from samples into sequencing libraries. It’s similar to DNA sequencing but has an extra step to convert the RNA to DNA.
It is commonly used in transcriptomic studies as a way to measure gene expression, which is important for understanding biological processes and disease.
Library preparation is the term used to describe the process of making a DNA/RNA/nucleic acid sample able to be sequenced. It’s a requirement for both short read and long read sequencing.
In order for a sequencer to be able to read the target DNA there has to be known specific synthetic DNA attached to the target DNA. These bits of DNA are known as sequencing adapters, and are usually supplied to the user as part of a sequencing kit. Once these are attached, the sample is part of a sequencing library. It’s call a library because it allows many different samples to be mixed and sequenced in parallel.
Depending on the application there can be other steps needed before the sequencing library is complete.
For example, the DNA may need to be made shorter and this is done by fragmentation – physically, using soundwaves, or chemically.
Library preparation is a crucial step in genome sequencing, involving several processes to prepare DNA or RNA samples for sequencing. Steps include DNR/RNA extraction, fragmentation, end repair and a-tailing, adapter ligation, size selection, PCR amplification, library quantification and quality control and sequencing. These steps ensure that the DNA or RNA is in the optimal format for high-quality, accurate sequencing data.
Next-generation sequencing library preparation involves a series of steps to convert DNA or RNA samples into a format compatible with sequencing. Initially, the nucleic acids are extracted and purified from the sample. The DNA or RNA is then fragmented into smaller pieces. For DNA sequencing, these fragments undergo end repair and A-tailing to create blunt ends and add an adenine overhang. Adapters, which are short DNA sequences necessary for the fragments to bind to the sequencing platform, are then ligated to the fragment ends. After this, size selection is performed to ensure a uniform fragment size range, followed by PCR amplification to increase the quantity of the library. The prepared library is quantified and assessed for quality before being loaded onto the sequencing platform for the sequencing process.
Potential issues found in library preparation for next-generation sequencing include: incomplete fragmentation, inefficient adapter ligation, PCR bias, contamination, and uneven sample representation, which can lead to poor sequencing quality and data inconsistency. Automation can mitigate these problems by providing precise and consistent execution of each step, reducing human error and contamination risks, and ensuring reproducibility.